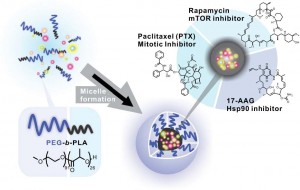
 Co-D Therapeutics’ lead product Triolimus, for the treatment of angiosarcoma, breast cancer and non-small cell lung cancer, is in preclinical development. Triolimus is a 3-in-1 nanomedicine that carries paclitaxel, rapamycin, and 17-AAG for combination cancer therapy (Fig. 3). Paclitaxel is the best selling anti-neoplastic agent of all time with broad application, but drug resistance is common. 2-drug combinations of paclitaxel and rapamycin(mTOR inhibitor) or of paclitaxel and 17-AAG(Hsp90 inhibitor) have entered clinical trials, seeking to overcome drug resistance, and results are promising. However, all three agents are poorly water-soluble, and they require an injectable formulation, e.g. Cremophor/ethanol and DMSO/lipid for paclitaxel and 17-AAG, respectively, which adds to the toxicity of drug combinations and lowers drug efficacy.
Co-D Therapeutics’ lead product Triolimus, for the treatment of angiosarcoma, breast cancer and non-small cell lung cancer, is in preclinical development. Triolimus is a 3-in-1 nanomedicine that carries paclitaxel, rapamycin, and 17-AAG for combination cancer therapy (Fig. 3). Paclitaxel is the best selling anti-neoplastic agent of all time with broad application, but drug resistance is common. 2-drug combinations of paclitaxel and rapamycin(mTOR inhibitor) or of paclitaxel and 17-AAG(Hsp90 inhibitor) have entered clinical trials, seeking to overcome drug resistance, and results are promising. However, all three agents are poorly water-soluble, and they require an injectable formulation, e.g. Cremophor/ethanol and DMSO/lipid for paclitaxel and 17-AAG, respectively, which adds to the toxicity of drug combinations and lowers drug efficacy.
Co-D Therapeutics’ polymeric micelle technology provides an injectable and a uniquely safe delivery vehicle for paclitaxel, rapamycin, and 17-AAG (all drugs are off-patent), addressing a major shortcoming in clinical practice for combination cancer therapy. Polymeric micelle technology eliminates toxic intravenous formulations required for paclitaxel, rapamycin, and 17- AAG, noting that most compounds in preclinical development are poorly water-soluble. Moreover, polymeric micelle technology may enhance drug delivery to solid tumors. Thus, polymeric micelle technology enables translation of promising anticancer combinations into phase I clinical trials as unique nanomedicines, including Triolimus, which has shown promising preclinical safety and efficacy in xenograft models as a 3-drug combination. Efficacy and toxicity studies in mouse models have been completed at the University of Wisconsin-Madison. Ongoing CMC studies and toxicity studies in rats are currently being conducted at UW-Madison. The studies are funded by the WARF Accelerator Program. Co-D Therapeutics anticipates filing for IND designation in Q3 of 2015 and enter Phase I clinical trial
in Q4 2015.
